Value Added Medicine
Design and Development
The Altus Advantage: Value Added Medicine Design
Today’s Value Added Medicines must provide clinically and commercially meaningful benefits to patients, providers and payers. And promoting benefits is only possible if they are written in the product label – which means high risk, high cost and lengthy clinical studies.
- The Altus Advantage: New Labeling Without New Clinical Studies
We design Value Added Medicines to generate new label claims from cost-effective, low risk pharmacokinetic studies only. - The Altus Advantage: Value Added Medicine Development
Our unique experience, hard won from years of developing our own Value Added Medicines, allows seamless and efficient identification, selection and development of partner opportunities.
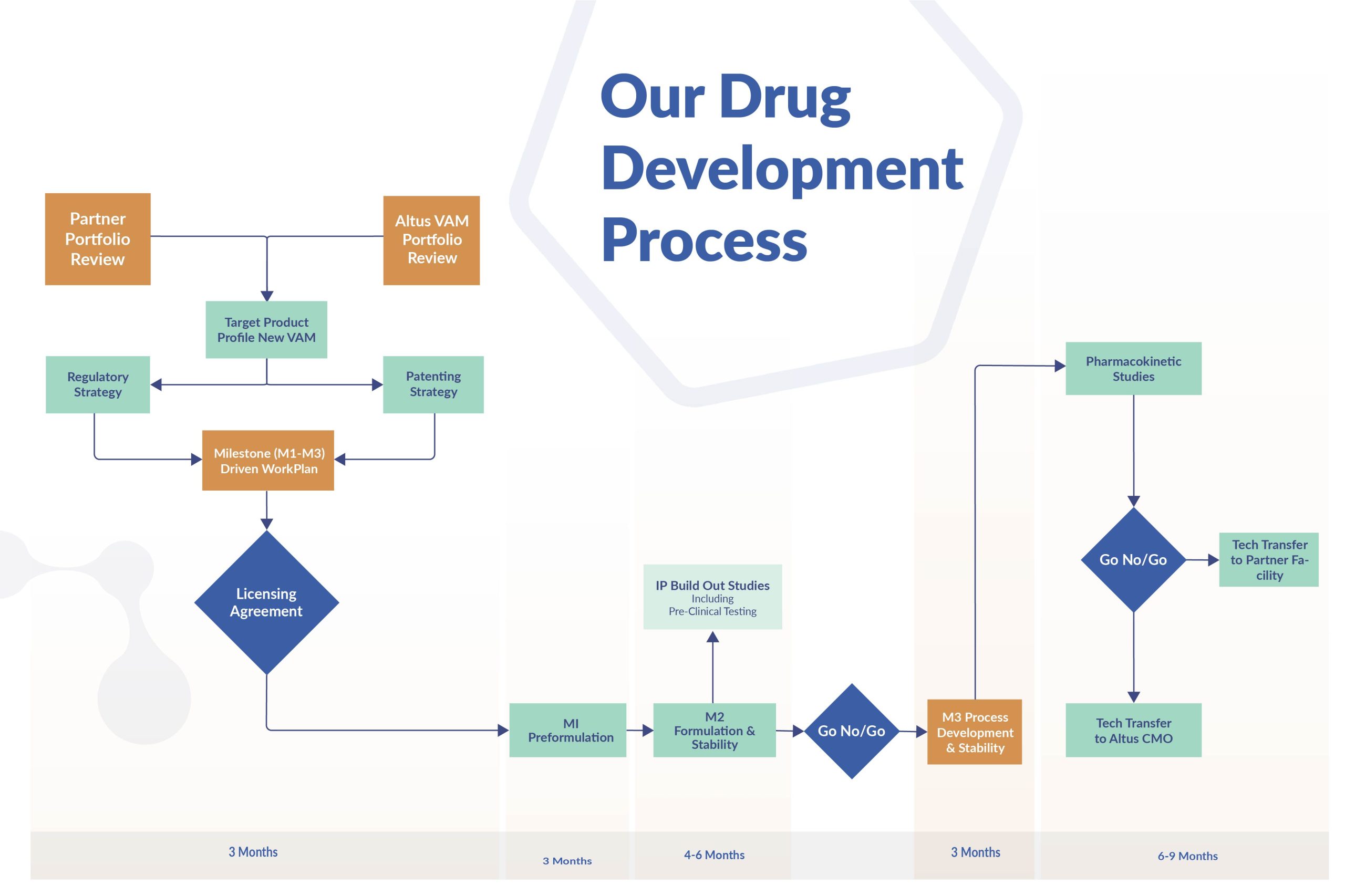
Regulatory Planning and Submission Strategy
Selecting the optimal regulatory submission strategy from the outset is crucial to designing a successful Value-Added Medicine. Not only will this strategy impact on market exclusivity for your new medicine, it will also dictate the economics of the project: selecting the wrong submission strategy results in wasted investment.
Regulations can change during development, so constant monitoring is essential. Missing a regulatory change can set development back years – or halt it altogether.
We offer a full range of regulatory intelligence and planning services to ensure the right choice from the start.
Planning the patent strategy for your pharmaceutical products
The Altus Patent Umbrella (link) provides market protection for all Value Added Medicines we develop. But Altus can offer more, filing new product specific applications early to cover each unique feature the new product will possess.